Heavy-Atom Ligation to 1st Row Transition Metals
Hard-soft theory in inorganic and main group chemistry provides that interactions between two “soft”, heavy atoms is favorable, while the interaction (binding, bonding) between one “soft” element and one “hard” element is unfavorable. While this is generally true, the utilization of polydentate ligands can be used to overcome such unfavorable dispositions.
A number of heavy transition metals such as gold (Au), platinum (Pt), rhodium (Rh), rhenium (Re) and iridium (Ir) are used in large scale energy-related chemical processes like H2 generation, C-H activation and carbon dioxide (CO2) reduction. And while these precious metals exhibit high catalytic activities, their high cost of use and recovery leads to high cost in chemical processing. In contrast, the heavy main group elements are relatively earth abundant and inexpensive.
Our goal is to simulate some of the spectroscopic and reactivity properties of heavy, precious metals by synthesizing a new class of complexes derived from heavy, main group elements bound to the late, 1st row transition metals (Mn, Fe, Co, Ni, Cu). In particular, we are exploring antimony and bismuth chemistry via the preparation of novel, polydentate Sb-M(3d) and Bi-M(3d) bonding motifs.
Related techniques and concepts include main group synthesis, transition metal insertion, X-ray crystallography, organometallic mechanisms, group theory, ligand field theory, and inorganic spectroscopy (UV/vis/NIR, EPR, NMR, magnetics/SQUID, DFT).
Relevant Publications
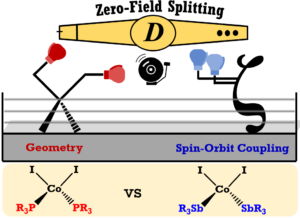
W. V. Taylor, B. K. Cashman, Z.-L. Xie, K. K. Ngo and M. J. Rose. Synthesis and Magnetic Properties of Antimony-Ligated Co(II) Complexes: Stibines versus Phosphines. Inorg. Chem. 2022, 61, 6733-6741. Link
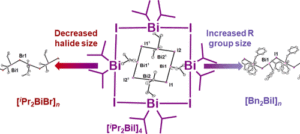
B. K. Cashman, K. M. Sandmann and M. J. Rose. Isolation and X-Ray Structure of Dialkylbismuth(III) Iodo “Nanosquare”: Breaking the Mold of Polymeric R2BiX. Inorg. Chem. 2019, 58, 13751-13754. Link
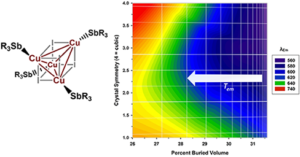
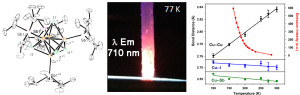
W. V. Taylor, C. X. Cammack, S. A. Shubert and M. J. Rose. Thermoluminescent Antimony-Supported Copper-Iodo Cuboids: Approaching NIR Emission via High Crystallographic Symmetry. Inorg. Chem. 2019, 58, 16330-16345. Link
**Invited Complementary Front Cover
**ACS Editor’s Choice 365 Selection

W. V. Taylor, Z.-L. Xie, N. I. Cool, S. A. Shubert, M. J. Rose. Syntheses, Structures and Characterization of Nickel(II) Stibines: Steric and Electronic Rationale for Metal Deposition. Inorg. Chem. 2018, 57, 10364-10374. Link
L. V. Taylor, U. H. Soto, V. M. Lynch and M. J. Rose. Antimony-Supported Cu4I4 Cuboid with Short Cu-Cu Bonds: Premise for NIR Thermoluminescence. Inorg. Chem. 2016, 55, 3206-3208. Link